Multiple clinical associations of MDS and autoimmunity have been described, but the pathogenesis and cause-effect relationship are likely diverse and possibly intertwining. Telomeropathies, while increasing risk of bone marrow failure, can be also associated with liver or lung fibrosis and therapies for Wegener's granulomatosis can predispose to MDS. Moreover, clonal hematopoiesis (CH) seems to be more prevalent in some autoimmune diseases 1 (AD) perpetuating a process that may ultimately lead to increased risk of MDS. Reverse scenarios are also possible and AD can be a result of MDS as a form of paraneoplastic syndrome. The recently established relationship of somatic UBA1 mutations and MDS with autoimmune manifestations exemplifies such a scenario e.g., VEXAS syndrome 2. This remarkable configuration prompted us to systematically investigate the relationships between autoimmune symptomatology and MDS including genomic features such as UBA1 mutations, pathomorphological phenotypes, and demographics.
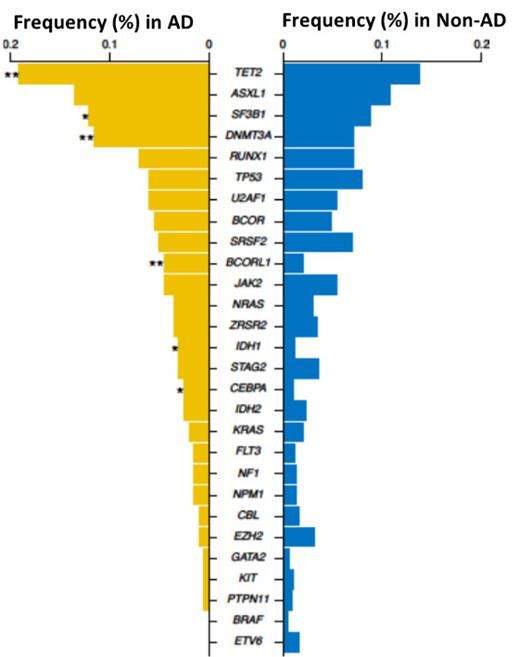
We have investigated 1,903 MDS patients for the presence and the anamnestic evidence of AD. A total of 197 patients (about 10%) had a concurrent well-defined AD (5.3%) or positive inflammatory/autoimmune markers (4.9%; fibromyalgia and chronic fatigue syndrome invoked 0.3% of MDS cases and were not counted). Of those, polymyalgia rheumatica, systemic lupus erythematosus and rheumatoid arthritis were most common accounting for 13%, 5.5% and 19% of MDS cases with autoimmunity. The remaining 60% involved the presence of serological markers such as ANA, anti-RNP and anti-SSA among others (45%, 4%, 2%, respectively) without fulfilling the criteria for specific autoimmune entities. The gender distribution of cases with MDS/autoimmune features was similar to the general MDS demographics and cases without autoimmune conditions. A similar distribution was seen among males and females in both groups, with males representing 84% of each subgroup and females accounting for 16%. When stratified by risk criteria, 58% of patients with MDS/AD were found to have low risk disease (<5% of blasts) compared to patients without AD (46%; p= .74), while intermediate to high risk (HR) categories were seen in 42% and 52% (p= .74), respectively. While trisomy 8 and complex karyotype were underrepresented in MDS with AD (15% vs 22%, 4.5% vs 10%), del(11q), del(12q) and del(20q) were more common among patients with AD (10% vs 5.8%). When we screened the genomic landscape of AD/MDS, we found more mutations in TET2 (24 vs 13%, p= .004), SF3B1 (14 vs 8%, p= .026), BCORL1 (5 vs 1.5%, p= .026), DNMT3A (13 vs 6%, p= .003), IDH1 (3 vs 0.8%, p= .01), and CEBPA (3 vs 0.8%, p= .04) compared to patients without AD ( Fig.1) With a median follow up of 19 months, female patients with AD showed better 1-year survival rate of 89% vs 65% in cases without AD, (p= .044). A similar trend was seen for transformation to AML. However, such differences were not found among males.
Next, we determined the etiologic fraction of the MDS/AD clinical dyad explained by the presence of VEXAS by screening UBA1 mutation in our cohort. We found mutations in 0.5% of MDS/AD vs 0.05% of the total cohort (p=0.1). To further expand on MDS association with AD, we investigated other potential autoimmune syndromes and found 0.5% of MDS with co-associated LGL with 1/3 of them presenting with RA. Notably, in the hypocellular MDS subgroup (n=175), 9.7% had concomitant AD (p= .89). Similarly, 0.3% of evaluable patients had Sweet syndrome, of which 16% associated with autoimmune conditions.
Finally, since AD appear to be frequent in MDS, we queried whether in our internal cohort of CHIP patients (n=115), their prevalence could be analogous. Indeed, AD were present in a fraction similar to that of manifest MDS (around 5%). Moreover, in all of these patients, similar to those with MDS, the autoimmune diagnosis preceded that of CHIP with the caveat that, unlike MDS, CHIP may have evolved much sooner than the original diagnosis but was not screened.
Our study provides valuable insights into the intricate relationship between MDS and AD. Our findings demonstrate that MDS and autoimmunity are linked through diverse mechanisms, with potential cause-effect relationships existing in both directions.
Figure 1. Frequency of mutations
Graph shows frequency of mutations among patients with autoimmune diseases (AD) in MDS versus those without AD.* p<0.05, **p<0.001.
Disclosures
Maciejewski:Regeneron: Consultancy, Honoraria; Alexion: Membership on an entity's Board of Directors or advisory committees; Novartis: Honoraria, Speakers Bureau; Omeros: Consultancy.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal